Транспортировка и хранение водорода
Водород обладает экологическими преимуществами по сравнению с природным газом и ископаемым топливом за счет отсутствия вредных выбросов. Однако для расширения масштабов производства необходимы эффективные технологии, надежные методы транспортировки и хранения, а также безопасные методы обращения

Углекислый газ в двух словах
- Ископаемое топливо используется во всех отраслях экономики, включая электроэнергетику, транспорт, промышленность, жилищное строительство и коммерческий сектор. Потребление ископаемого топлива и связанные с ним выбросы углекислого газа можно сократить, сжигая водород вместо ископаемого топлива или смешивая его с ним в некоторых областях применения.
- Водород создает уникальные факторы риска и является чрезвычайно огнеопасным, особенно при наличии кислорода, поэтому его транспортировка и хранение должны осуществляться безопасно с использованием надлежащих методов проектирования, монтажа, эксплуатации и технического обслуживания.
- Водород можно транспортировать по трубопроводам, грузовым и железнодорожным транспортом, а также с помощью морского судна. Оптимальный способ транспортировки зависит от количества перевозимого водорода, срока, в течение которого необходимо его доставить, расстояния, которое необходимо преодолеть, расположения транспортной инфраструктуры и его доступности.
- Водород можно хранить физически в виде газа, жидкости или их комбинации. Разрабатываются методы хранения водорода в различных составах и материалах, таких как жидкая органика, гидриды металлов и адсорбенты, или в сочетании с ними. Кроме того, ученые изучают возможности его хранения в соляных пещерах.
- Различия между ископаемым топливом и водородом достаточно велики, поэтому необходимо провести специальное обучение персонала, занимающегося проектированием, установкой, эксплуатацией и обслуживанием систем хранения и транспортировки водорода.
Вызов
Ископаемое топливо является источником энергии для большинства современных объектов инфраструктуры, но при его сжигании выделяются загрязняющие вещества, в первую очередь углекислый газ. Это парниковый газ (ПГ), который считается причиной глобального потепления и изменения климата.
И наоборот, при сжигании водорода образуется безвредный водяной пар и некоторое количество оксида азота (NOx), при этом не выделяется диоксид углерода и другие загрязняющие вещества, такие как диоксид серы (SOx). Кроме того, водород совместим со многими существующими газовыми турбинами с двигателями внутреннего сгорания, которые могут работать на водороде, природном газе или их сочетании. Однако при неумелом обращении водород представляет собой опасное вещество.
Во-первых, его молекулы являются самыми маленькими из всех элементов, поэтому утечка из резервуаров и трубопроводов, которая создает опасность пожара и взрыва, представляет собой серьезную проблему. Особое внимание следует уделить материалам и методам, которые применяются для герметизации этих систем, например, фитингам, прокладкам, клапанам и другим уплотнительным устройствам. Для обнаружения нештатных ситуаций, таких как потеря герметичности, должны применяться устройства мониторинга окружающей среды, такие как детекторы пламени и горючих газов, или встроенные устройства, такие как датчики давления и температуры. Поскольку водород является двухатомным, более новые технологии, такие как инфракрасные газовые детекторы, часто используемые для обнаружения природного газа, не могут быть использованы для обнаружения водородного газа.
Результаты анализа
Молекулы водорода являются самыми маленькими из всех элементов, поэтому утечка из резервуаров и трубопроводов, которая создает опасность пожара и взрыва, представляет собой серьезную проблему.
Утечки в основном являются результатом охрупчивания, которое чаще всего возникает при поглощении сталью и другими металлами атомов водорода. Эти атомы могут соединяться, образовывая молекулы водорода, которые распространяются по всему металлу и образуют пузырьки, ослабляющие материал, вызывая охрупчивание и растрескивание даже при температуре окружающей среды. Поэтому очень важно смягчить эти проблемы путем выбора подходящих материалов в зависимости от области применения.
Результаты анализа
Водород может вызвать катастрофический отказ оборудования из-за разъедания металлических труб, резервуаров и других защитных механизмов, а также клапанов, фитингов, прокладок и других уплотнений.
Хранение водорода
Безопасное хранение водорода — ключевой фактор, способствующий развитию водородных технологий и технологий топливных элементов.

Резервуары для хранения водорода
Водород можно хранить в виде сжатого газа или криогенной жидкости. Сжатый газообразный водород обычно хранится в резервуарах под давлением 350-700 бар (5 000-10 000 фунтов на квадратный дюйм). Полностью жидкий водород можно хранить при температуре -253 °C (-423 °F), а криокомпрессионный водород — при температуре -233 °C (-387 °F). Газообразные хранилища требуют меньше оборудования и значительно экономичнее, но у жидких хранилищ есть свои преимущества, в первую очередь гораздо более высокая плотность хранения энергии
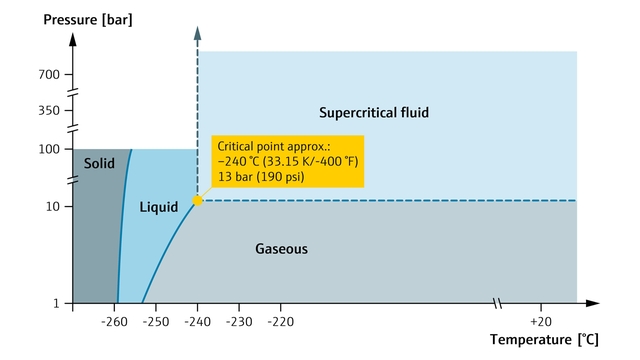
Фазы водорода в зависимости от давления и температуры.
Жидкий водород уже давно используется в качестве ракетного топлива для запусков космических аппаратов. В космосе он хранится в виде сжатого газа или криогенной жидкости в баллонах, трубках и сферических резервуарах. В газообразном состоянии водород обычно хранится в баллонах. Однако для хранения жидкого водорода предпочтительнее использовать сферические резервуары, чтобы минимизировать площадь поверхности, которая напрямую связана с теплоотдачей от окружающей среды.
Водород также может храниться в системах на основе материалов на поверхности твердых тел (адсорбция) или внутри них (абсорбция). Эти процедуры разрабатываются для соблюдения требований к плотности топлива и повышения безопасности процесса, поскольку они снижают вероятность утечек и неконтролируемого горения.
Меры безопасности для всех систем хранения водорода включают:
- Хранить нужно в хорошо проветриваемых местах на открытом воздухе, вдали от строений, транспортных средств, тепла, источников искр и открытого пламени. Курить в таких местах запрещено
- Никогда не перетаскивайте, не катите, не сдвигайте и не роняйте контейнеры для хранения
- При работе с водородом используйте только искробезопасные инструменты и взрывозащищенное оборудование
- Заземлите все оборудование и трубопроводы
- Регулярно проверяйте герметичность водородных систем с помощью мыльного раствора, но ни в коем случае не с помощью пламени
Хранение водорода в транспортных средствах
Требования к хранению водорода высокой плотности создают серьезные проблемы для транспортных систем. Плотность энергии водорода гораздо ниже, чем у бензина, поэтому для хранения того же количества энергии требуются более крупные резервуары. Как правило, автомобильные водородные баллоны больше, чем баллоны для природного газа, так как они способны выдерживать более высокое давление.
Эти дополнительные требования к габаритам автомобиля снижают его функциональность, позволяя с комфортом перевозить людей и предметы в условиях ограниченного пространства, а лишний вес негативно сказывается на расстоянии, которое автомобиль может проехать, затратив определенное количество энергии. Кроме того, водородные топливные элементы занимают больше места, чем двигатели внутреннего сгорания, увеличивают вес и создают еще один потенциальный источник утечки.
Легковые и грузовые автомобили, работающие на водороде, уже выпускаются, но количество водородных заправочных станций во всем мире невелико. Из-за этого они непрактичны для большинства людей, особенно для повседневных потребителей. Однако по мере развития водородной инфраструктуры эта реальность может измениться.
Несмотря на эти недостатки, легковые и грузовые автомобили, работающие на водороде, имеют значительные преимущества по сравнению с электромобилями. Их можно заправить за несколько минут, а не за несколько часов, и запасенная энергия не разрушается со временем. Плотность хранения энергии намного выше, чем у аккумуляторов, более чем в 100 раз, что делает топливо намного легче и компактнее, чем аккумуляторы. И наконец, материалы, необходимые для производства современных аккумуляторов, особенно литий, в дефиците, в то время как для производства водородных топливных элементов материалов в избытке.
Транспортировка водорода
При производстве газообразного водорода его можно потреблять на месте, подвергать сжатию и транспортировать в близлежащие резервуары или заполнить им баллоны для транспортировки, а также подвергать сжижению для повышения плотности хранения или транспортировки на большие расстояния. Транспортировка водорода обычно осуществляется по трубопроводам, грузовым, железнодорожным или морским транспортом. Чаще всего трубопроводы используются между близлежащими производственными объектами и потребителями, а также в более широких географических зонах, где прогнозируется стабильный долгосрочный спрос.
Результаты анализа
Водород можно транспортировать в виде газа, жидкости или в сочетании обоих видов, причем для каждого формата применяются отдельные системы безопасности.
На коротких расстояниях чаще всего используется грузовой транспорт, который перевозит удлиненные баллоны высокого давления, уложенные в трубчатый прицеп, или танкеры с жидким водородом при криогенных температурах. Железнодорожные вагоны используются для перевозки жидкого водорода на средние расстояния, а для транспортировки тяжелых грузов на дальние расстояния используются морские суда.

Транспортировка водорода с помощью грузового транспорта.
Навстречу будущему
Продолжаются исследования, направленные на разработку жизнеспособных компактных систем хранения водорода, безопасных для использования как в транспортных средствах, так и в стационарных установках. В сочетании с эффективным производством водорода, разработки в области транспортировки и хранения будут способствовать развитию водородной экономики.
Поскольку в промышленности наблюдается тенденция к сокращению выбросов углекислого газа путем внедрения водорода и других альтернативных видов топлива в свою инфраструктуру, необходимо обеспечить надлежащую подготовку кадров для безопасного проектирования, установки, эксплуатации и обслуживания этих систем.



